
At sufficiently high pressure above the critical point, the gas will have the density of a liquid but will not condense. There is a critical point-that is, a critical temperature-above which liquid cannot exist. At lower temperatures, the curves begin to look less like hyperbolas-the gas is not behaving ideally and may even contain liquid. The hyperbolas represent ideal-gas behavior at various fixed temperatures, and are called isotherms. Figure 2 shows a graph of pressure versus volume.

If you plot the relationship PV = constant on a PV diagram, you find a hyperbola. Now, assuming the number of molecules and the temperature are fixed, PV = constant (ideal gas, constant temperature).įor example, the volume of the gas will decrease as the pressure increases. When the substance behaves like an ideal gas, the ideal gas law describes the relationship between its pressure and volume. We can examine aspects of the behavior of a substance by plotting a graph of pressure versus volume, called a PV diagram.

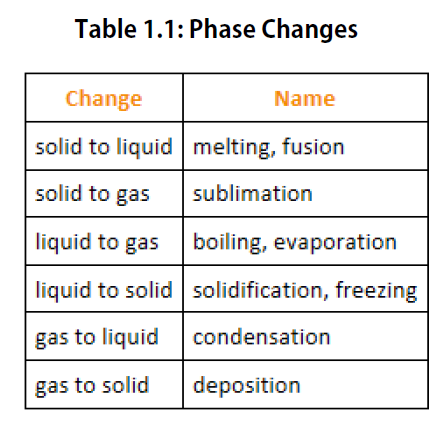
In dermatology, LN 2 is used to freeze and painlessly remove warts and other growths from the skin. It is also used to reduce noise in electronic sensors and equipment, and to help cool down their current-carrying wires. LN 2 is useful as a refrigerant and allows for the preservation of blood, sperm, and other biological materials. It boils at 77 K ( –196✬) at atmospheric pressure. LN 2 is made by liquefaction of atmospheric air (through compression and cooling). Solid CO 2 is called “dry ice.” Another example of a gas that can be in a liquid phase is liquid nitrogen (LN 2). If the pressure is reduced, the temperature drops and the liquid carbon dioxide solidifies into a snow-like substance at the temperature –78✬. Carbon dioxide, for example, is a gas at room temperature and atmospheric pressure, but becomes a liquid under sufficiently high pressure. High pressure may also cause a gas to change phase to a liquid. The volume decreases slightly once the substance is solid, but it never becomes zero. When the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. The linear (straight line) part of the graph represents ideal gas behavior-volume and temperature are directly and positively related and the line extrapolates to zero volume at –273.15✬, or absolute zero. A sketch of volume versus temperature for a real gas at constant pressure. The volume never reaches zero because of the finite volume of the molecules. When a liquid is cooled to even lower temperatures, it becomes a solid. The substance changes from a gas to a liquid. The molecules are very close (condensation occurs) and there is a dramatic decrease in volume, as seen in Figure 1. At lower temperatures, however, the interactions between the molecules and their volumes cannot be ignored. Real gases are like ideal gases at high temperatures. Up to now, we have considered the behavior of ideal gases. Describe the state of equilibrium between a liquid and a gas, a liquid and a solid, and a gas and a solid.Identify and describe the triple point of a gas from its phase diagram.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
